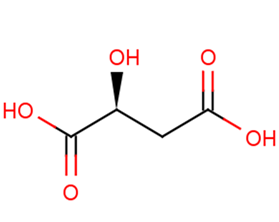
L-(-)-Malic acid
CAS No. 97-67-6
L-(-)-Malic acid( (S)-2-Hydroxysuccinic acid | (S)-(-)-HYDROXYSUCCINIC ACID )
Catalog No. M19676 CAS No. 97-67-6
Malic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid which contributes to the sourness of a green apple. Malic acid can make a wine taste tart although the amount decreases with increasing fruit ripeness.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 100MG | 37 | In Stock |


|
| 500MG | 86 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameL-(-)-Malic acid
-
NoteResearch use only, not for human use.
-
Brief DescriptionMalic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid which contributes to the sourness of a green apple. Malic acid can make a wine taste tart although the amount decreases with increasing fruit ripeness.
-
DescriptionMalic acid is a tart-tasting organic dicarboxylic acid that plays a role in many sour or tart foods. Apples contain malic acid which contributes to the sourness of a green apple. Malic acid can make a wine taste tart although the amount decreases with increasing fruit ripeness. (wikipedia). In its ionized form malic acid is called malate. Malate is an intermediate of the TCA cycle along with fumarate. It can also be formed from pyruvate as one of the anaplerotic reactions. In humans malic acid is both derived from food sources and synthesized in the body through the citric acid cycle or Krebs cycle which takes place in the mitochondria. Malate's importance to the production of energy in the body during both aerobic and anaerobic conditions is well established. Under aerobic conditions the oxidation of malate to oxaloacetate provides reducing equivalents to the mitochondria through the malate-aspartate redox shuttle. During anaerobic conditions where a buildup of excess of reducing equivalents inhibits glycolysis malic acid's simultaneous reduction to succinate and oxidation to oxaloacetate is capable of removing the accumulating reducing equivalents. This allows malic acid to reverse hypoxia's inhibition of glycolysis and energy production. In studies on rats it has been found that only tissue malate is depleted following exhaustive physical activity. Other key metabolites from the citric acid cycle needed for energy production were found to be unchanged. Because of this a deficiency of malic acid has been hypothesized to be a major cause of physical exhaustion. Notably the administration of malic acid to rats has been shown to elevate mitochondrial malate and increase mitochondrial respiration and energy production.
-
In VitroIt is showed that ME is essential for (S)-2-Hydroxysuccinic acid (L-malic acid) utilization in L. casei. Furthermore, deletion of either the gene encoding the histidine kinase or the response regulator of the TC system resulted in the loss of the ability to grow on (S)-2-Hydroxysuccinic acid, thus indicating that the cognate TC system regulates and is essential for the expression of ME. Transcriptional analyses shows that expression of maeE is induced in the presence of (S)-2-Hydroxysuccinic acid and repressed by glucose, whereas TC system expression is induced by (S)-2-Hydroxysuccinic acid and is not repressed by glucose.
-
In Vivo——
-
Synonyms(S)-2-Hydroxysuccinic acid | (S)-(-)-HYDROXYSUCCINIC ACID
-
PathwayProteasome/Ubiquitin
-
TargetEndogenous Metabolite
-
Recptorothers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number97-67-6
-
Formula Weight134.09
-
Molecular FormulaC4H6O5
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESO[C@@H](CC(O)=O)C(O)=O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Sreekumar A Poisson L M Rajendiran T M et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.[J]. Nature 2009 457(7231):910-914.
molnova catalog



related products
-
5'-Guanylic acid
5'-Guanylic acid is involved in several metabolic disorders, including AICA-ribosiduria pathway, ?adenosine deaminase deficiency,adenine phosphoribosyltransferase deficiency (aprt), and the 2-hydroxyglutric aciduria pathway.
-
Aprocitentan
Aprocitentan is ETA and ETB antagonist .
-
Docosapentaenoic aci...
Docosapentaenoic acid 22n-3 is present in the cell membranes of all animals and is a component of phospholipids.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


